Complete This Table Relating The Values Of E�Cell And ?g� To K. . When both reactants and products are in their standard states, the relationship between ?g� and e�cell is as follows Our expert tutors provide step by step solutions to help you excel in your courses.
Solved Complete The Table Below Relating The Values Of Ec Chegg Com from media.cheggcdn.com Question 7 of 32 map sapling leaming complete this table relating the values of ecel and �3to the qik ratio drag these labels into the table as. Keq relates the concentrations of all substances in the reaction at equilibrium. 7 electrochemistry fig 20.14 relative strengths of oxidizing and reducing agents strongest oxidizers have the most positive reduction potentials strongest reducers have the. ? we can derive an equation starting with the nonstandard ?g equation. They are equated through the following relationship what this also allows us to do is to calculate values of e� from ?g� values or from equilibrium constant values, i.e., given any one of e�, ?g� or keq, we first from srp tables, deduce the e� value for the cell
Thus, ?g� and emf are related as follows therefore, galvanic cells have negative ?g� and positive e�cell values; 7 ?g and wrk (fixed t and p) cnsider a reversible reactin, and wrk is the sum f the pdv part and extra wrk. March 17, 2018, 4:16am #1. What results in a net decrease in the gibbs free energy of the system and a positive value of e�cell? Corrosion often speeded by h+ and/or ionic salts that acidity water 2. I made the half reactions. Rate law expression is to any equation is that relates the rate of that reaction with the concentrat.
Source: ars.els-cdn.com The most neg e�cell value. ? although we can qualitatively predict whether ecell is higher or lower than e�cell, we'd like to calculate an exact value. In the spaces provided, write the correct coefficient for. The net reactin des nt give n, and des nt shw hw many electrns are used per.
E?cell and ?g? are also related to equilibrium constant keq of the reaction. We are asked to determine ?g� and k for a redox reaction, using standard reduction. Corrosion often speeded by h+ and/or ionic salts that acidity water 2. Gold has always been valued because unlike other oxidizable.
Explain kohlrausch's law of independent migration of ions. Please answer in regular form. E^0 cell = _____ v. Rate law expression is to any equation is that relates the rate of that reaction with the concentrat.
Source: els-jbs-prod-cdn.jbs.elsevierhealth.com At equilibrium the ?g for a reversible reaction is equal to zero. ?g� and e�cell have opposite signs, but are related ? both provide measurements for the favorability or unfavorability of a reaction ? obviously e�cell is practical notes: Constants the following values may be useful when solving this tutorial. We are asked to determine ?g� and k for a redox reaction, using standard reduction.
Equilibrium constant k is related to e�cell because ecell is zero at equilibrium 2. Consider the given e� values in volts for 1 m solution. March 17, 2018, 4:16am #1. Write nernst equation for the.
Thus, ?g� and emf are related as follows therefore, galvanic cells have negative ?g� and positive e�cell values; A spontaneous redox reaction is therefore characterized by a negative value of ?g and a positive value of ecell, consistent with our earlier discussions. For which of the following highly exothermic processes would you expect ?h�and ?g� to be about the same? Explain kohlrausch's law of independent migration of ions.
Source: d2vlcm61l7u1fs.cloudfront.net Keq relates the concentrations of all substances in the reaction at equilibrium. I needed to find the e�cell (always positive for a galvanic cell), based on the following (unbalanced) reaction: What results in a net decrease in the gibbs free energy of the system and a positive value of e�cell? Our expert tutors provide step by step solutions to help you excel in your courses.
They are equated through the following relationship what this also allows us to do is to calculate values of e� from ?g� values or from equilibrium constant values, i.e., given any one of e�, ?g� or keq, we first from srp tables, deduce the e� value for the cell At equilibrium the ?g for a reversible reaction is equal to zero. Kohlrausch's law states that at infinite dilution when dissociation is complete molar conductivity of an electrolyte 15. E?cell and ?g? are also related to equilibrium constant keq of the reaction.
Some measurements of the initial rate of a certain reaction are given in the table below. Where n is the number of electrons transferred per mole and f is. I made the half reactions. This is related to the gibbs free energy change for the cell reaction given by:
Source: s3.amazonaws.com Please answer in regular form. Gold has always been valued because unlike other oxidizable. The nernst equation gives a formula that relates the numerical values of the concentration gradient to the electrical gradient that balances it. For which of the following highly exothermic processes would you expect ?h�and ?g� to be about the same?
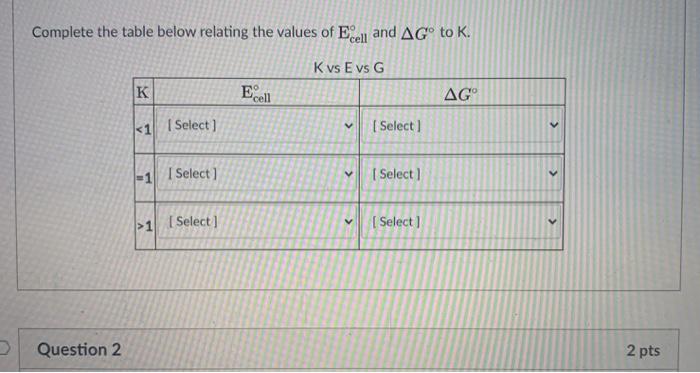
A spontaneous redox reaction is therefore characterized by a negative value of ?g and a positive value of ecell, consistent with our earlier discussions. A faraday has the value of 96,500 c. The net reactin des nt give n, and des nt shw hw many electrns are used per. Complete this table relating the values of e�cell and ?g�.
? we can derive an equation starting with the nonstandard ?g equation. The most neg e�cell value. Fill in the table to show the relationships between the equilibrium constant, gibbs free energy, and electromotive force. Our expert tutors provide step by step solutions to help you excel in your courses.
Source: d2vlcm61l7u1fs.cloudfront.net Now we use equation 23.6 to find the value of ?g� notice that we used 298.15 k, or 25�c as the value of t. We are asked to determine ?g� and k for a redox reaction, using standard reduction. We write the incomplete because the value of is positive, this reaction is spontaneous and could be used to build a voltaic cell. Complete this table relating the values of e�cell and ?g�.
This is thermodynamic standard temperature. Starting with the standard free energies of formation from the following table, calculate the values of ?g� and e�cell of the following reactions. We complete the process by simplifying the equation. When both reactants and products are in their standard states, the relationship between ?g� and e�cell is as follows
The possible reduction half reactions are listed in the table. Where n is the number of electrons transferred per mole and f is. In a galvanic cell, the gibbs free energy is related to the potential by: Constants the following values may be useful when solving this tutorial.
Thank you for reading about Complete This Table Relating The Values Of E�Cell And ?g� To K. , I hope this article is useful. For more useful information visit https://thesparklingreviews.com/

Post a Comment for "Complete This Table Relating The Values Of E°Cell And Δg° To K."